In industriellen Kühlsystemen kommen verschiedene Metalle zum Einsatz, jedes mit spezifischen Eigenschaften und Vorteilen. Die Auswahl und Anwendung der richtigen chemischen Behandlungsmittel sind entscheidend, um Korrosion zu verhindern, Ablagerungen zu minimieren und die Effizienz der Systeme zu maximieren. Dieser Artikel beleuchtet die gängigsten Metalle in Kühlsystemen, ihre Eigenschaften und die chemischen Maßnahmen, die ergriffen werden müssen, um sie zu schützen.
Inhaltsverzeichnis
Bedeutung der Metalle in Kühlsystemen
Metalle spielen eine zentrale Rolle in Kühlsystemen aufgrund ihrer Wärmeleitfähigkeit, mechanischen Stabilität und Langlebigkeit. Die richtige Auswahl und Behandlung der Metalle sind entscheidend, um Korrosion zu verhindern und die Effizienz des Kühlsystems zu gewährleisten.
Typische Metalle in Kühlsystemen
Stahl
Stahl ist weit verbreitet in industriellen Kühlsystemen, insbesondere in Rohrleitungen und Behältern. Stahl bietet eine gute mechanische Festigkeit, ist jedoch anfällig für Korrosion, insbesondere in Anwesenheit von Sauerstoff und Chloriden.
Kupfer
Kupfer wird häufig in Wärmetauschern verwendet, da es eine ausgezeichnete Wärmeleitfähigkeit bietet. Allerdings ist Kupfer korrosionsanfällig in sauren und alkalischen Umgebungen sowie bei Kontakt mit Ammoniak und Schwefelverbindungen.
Aluminium
Aluminium findet in Kühltürmen und Verdampfern Anwendung, da es leicht und korrosionsbeständig ist. Es reagiert jedoch empfindlich auf alkalische Lösungen und chloridhaltige Umgebungen.
Edelstahl
Edelstahl wird in Kühlsystemen eingesetzt, wo hohe Korrosionsbeständigkeit erforderlich ist. Er bietet eine hervorragende Beständigkeit gegenüber einer Vielzahl von chemischen Angriffen, ist jedoch teurer als andere Materialien.
Chemische Behandlung und Schutzmaßnahmen
Korrosionsinhibitoren
Korrosionsinhibitoren sind essenziell, um Metalloberflächen in Kühlsystemen zu schützen. Sie bilden eine schützende Schicht, die das Metall vor oxidativen Angriffen bewahrt. Typische Korrosionsinhibitoren umfassen Phosphate, Silikate und organische Verbindungen. Wichtig ist, dass bestimmte Inhibitoren für spezifische Metalle ungeeignet sein können.
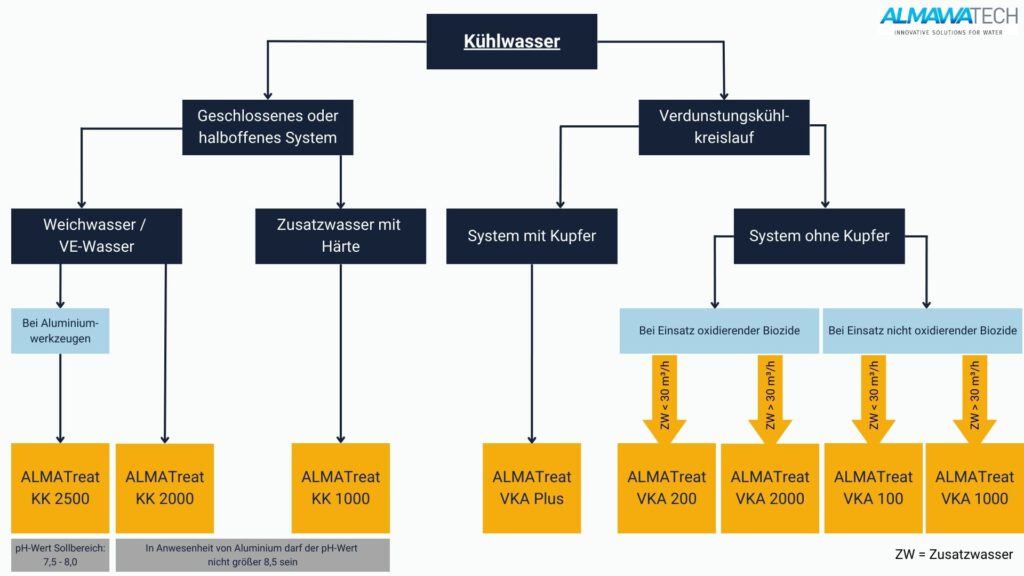
Foto: Hier finden Sie eine grobe Übersicht unserer Betriebsmittel für Kühlsysteme sowie eine Orientierungshilfe zur Unterteilung der Produktgruppen nach Anwendung und Aufgabenstellung.
Härtestabilisatoren
Härtestabilisatoren verhindern die Ausfällung von Calciumcarbonat und anderen mineralischen Ablagerungen. Dies ist besonders wichtig in Systemen mit hohen Temperaturen und Wasserhärten, um die Effizienz der Wärmeübertragung zu erhalten.
Dispergiermittel
Dispergiermittel halten Schwebstoffe in Schwebe und verhindern deren Ablagerung. Sie sind wichtig, um die Ansammlung von Schmutz, Korrosionsprodukten und biologischem Material zu verhindern.
Biozide
Biozide werden eingesetzt, um das Wachstum von Mikroorganismen wie Bakterien, Algen und Pilzen zu kontrollieren. Sie verhindern biologisches Fouling und schützen das System vor biologischen Angriffen.
Exemplarische Behandlung einiger Metalle und chemischer Verbindungen
Stahl und seine Behandlung
Stahl ist anfällig für allgemeine und Lochkorrosion. Die Verwendung von Korrosionsinhibitoren wie Nitriten und Phosphaten kann Stahl wirksam schützen. Dispergiermittel helfen, die Ablagerung von Korrosionsprodukten zu verhindern, während Biozide das biologische Wachstum kontrollieren. Phosphate sollten jedoch in Systemen mit hohem Sauerstoffgehalt vermieden werden, da sie zu Ablagerungen führen können.
Kupfer und seine Behandlung
Kupfer kann durch Sauerstoff, Schwefelverbindungen und Ammoniak korrodieren. Benzotriazol und Tolytriazol sind wirksame Korrosionsinhibitoren für Kupfer, die eine Schutzschicht auf der Kupferoberfläche bilden. Härtestabilisatoren und Biozide ergänzen den Schutz. Chlorhaltige Biozide sollten jedoch vermieden werden, da sie die Korrosion von Kupfer beschleunigen können.
Aluminium und seine Behandlung
Aluminium ist korrosionsbeständig, aber empfindlich gegenüber alkalischen Umgebungen und Chloriden. Silikatinhibitoren bieten Schutz, indem sie eine Schutzschicht auf der Aluminiumoberfläche bilden. Es ist wichtig, den pH-Wert des Wassers zu kontrollieren und Dispergiermittel zu verwenden, um Ablagerungen zu verhindern. Phosphate und stark alkalische Härtestabilisatoren sollten vermieden werden, um Korrosion zu verhindern.
Edelstahl und seine Behandlung
Edelstahl ist widerstandsfähig gegenüber vielen chemischen Angriffen, aber in chloridhaltigen Umgebungen anfällig für Spannungsrisskorrosion. Molybdänhaltige Inhibitoren können zusätzlichen Schutz bieten. Die regelmäßige Anwendung von Bioziden verhindert biologisches Fouling, während Härtestabilisatoren und Dispergiermittel Ablagerungen und Schmutz kontrollieren. Chloride sollten minimiert werden, um Spannungsrisskorrosion zu vermeiden.
Zusammenfassung und Empfehlungen
Die Auswahl des richtigen Metalls und der entsprechenden chemischen Behandlung ist entscheidend für den effizienten und langlebigen Betrieb von Kühlsystemen. Korrosionsinhibitoren, Härtestabilisatoren, Dispergiermittel und Biozide spielen eine zentrale Rolle bei der Pflege und dem Schutz dieser Systeme. Durch eine gezielte chemische Behandlung können Korrosion, Ablagerungen und biologische Kontaminationen wirksam verhindert werden.
Unser Ziel ist es, den Betrieb Ihrer Kühlsysteme so effizient und reibungslos wie möglich zu gestalten. Erfahren Sie mehr über unsere umfassenden Lösungen und wie sie Ihnen helfen können, die Leistung und Lebensdauer Ihrer Kühlsysteme zu maximieren. Für eine maßgeschneiderte Beratung und optimale Dosierungslösungen stehen wir Ihnen gerne zur Verfügung.

Unsere Produktreihe ALMA AQUA – Effiziente Betriebsmittel für Kühlkreisläufe
Kostenlose Beratungstermine
Vereinbaren Sie einen Online-Beratungstermin mit unserm Spezialisten für die Aufbereitung von Kühlwasser.